Greenlight Guru serves medical device teams that manage documents, design work, and compliance activity in a regulated environment.
Many organizations start with the platform during the early stages. As their quality systems become more complex, some teams notice limitations in workflow choices or in collaboration across groups.
These limits can appear during audits or during new product work that places more pressure on the system.
This article explains why companies explore other platforms and what they look for during that search. We’ll also provide a list of leading Greenlight Guru competitors.
What Is Greenlight Guru?
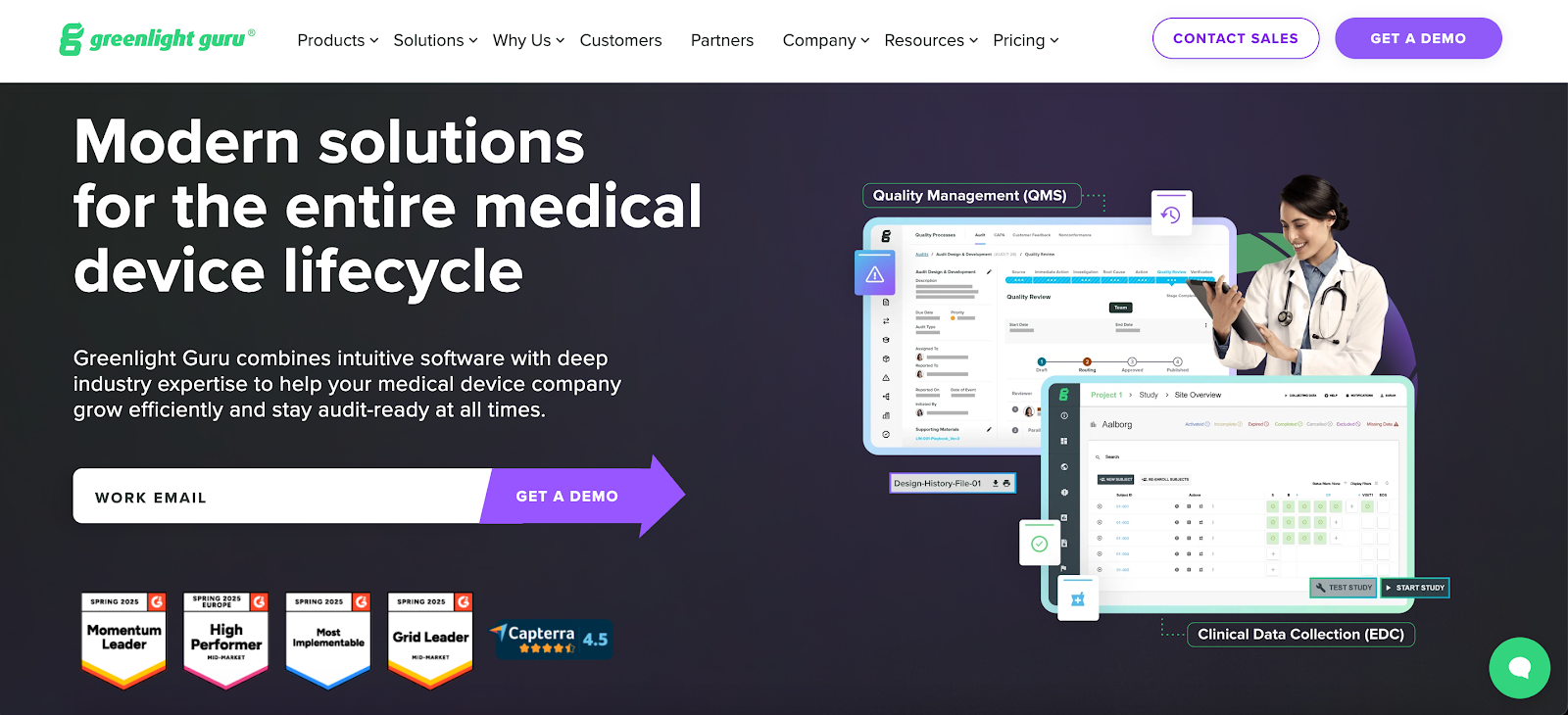
Image source: greenlight.guru
Greenlight Guru is a quality management system (QMS) for medical device companies. It organizes documents, design controls, risk files, and quality events in one place.
It also supports the Food and Drug Administration (FDA) and the International Organization for Standardization (ISO) requirements, which help teams create and maintain compliant records.
You’ll find dedicated areas for design work, change activity, training, and audits.
Many teams like the built-in traceability between design controls and risk activity. They also note that the layout is simple enough for new participants to understand without extensive learning.
These points make it a common starting choice for small or early-stage groups.
Why Companies Look for Greenlight Guru Alternatives
Many teams start with Greenlight Guru during the early stages. As their quality system expands, they often notice limits that affect daily work.
These points capture the most common concerns users have with Greenlight Guru.
- Rigid workflows: Teams can’t adjust required steps to match internal procedures. This becomes more noticeable when compliance reviews require a different sequence of actions.
- Inconsistent workspace behavior: Some areas save fields with one click. Other areas require extra steps. These differences slow review work and increase the chance of mistakes during audits.
- Limited data handling: Greenlight Guru doesn’t support bulk uploads. Teams with large document sets need to upload files in small batches. This leads to long setup periods and slow onboarding.
- Restricted integration: Some companies need direct integration with manufacturing or enterprise resource planning (ERP) systems. They want quality records to link to production data or supplier information. Greenlight Guru provides only basic support in these areas.
- Narrow scalability: The platform becomes harder to use once more departments need access to documents, training records, and quality events.
These issues often push companies to look for platforms that support a wider set of quality tasks and better coordination across departments.
5 Leading Alternatives to Greenlight Guru
These alternatives regularly surface when teams conduct a QMS evaluation. Each platform covers a different set of functions, such as document control, training records, or production links.
1. TLM

Total Lean Management (TLM) gives medical device teams a complete quality management software system for ISO 9001 and ISO 13485 needs.
It manages audits, corrective and preventive action (CAPA) work, documents, training records, supplier files, and production data in one environment.
You can run it in the cloud or on-premises with the same function set.
Many companies review TLM as a top Greenlight Guru competitor when they need more control over configuration.
TLM adapts to unique processes, so teams avoid workarounds that slow daily work. This is one of the main reasons buyers place it among the best Greenlight Guru alternatives.
Key Features
- 30+ modules across quality, risk, production, and IT security
- Custom fields and renamable modules for company-specific terminology
- Build your own Custom Forms with email notification and approvals
- AI-Powered compliance reviews and document content searching
- System settings that add options without breaking current setups
- Optional development work for requirements that fall outside base modules
- TLM Dashboard that gathers tasks from every module
- Reporting sections for metrics and trend review
- Support for multiple locations with record separation
- Cloud or on-premises deployment
- Connection Manager for ERP, QuickBooks, Zoho, and other systems
- Template procedures for ISO standards
- Validation document packages for ISO 13485
- Multi-factor authentication with U.S.-based security
How TLM Works
TLM uses two applications that serve different groups. The main application supports managers who build workflows, configure fields, approve changes, and control document activity.
The companion web app supports staff who complete assigned tasks, read documents, confirm training, and approve routine items.
This structure keeps advanced screens in the main application. Staff work only in the areas needed for their role. It keeps work specific to each user type.
What Sets TLM Apart From Greenlight Guru
TLM gives teams configuration options that support company-specific procedures. Fields and modules can be adjusted to match internal terminology.
Workflow steps can be set in a sequence that fits existing procedures. These options help teams maintain a consistent record flow during audits and review cycles.
TLM also combines quality tasks, production activity, and product lifecycle management (PLM) records in one suite. The system covers audits, CAPA, document control, training, supplier oversight, equipment tracking, integrated FMEA and control plans, and product data.
Companies can manage this work in one environment instead of jumping between separate apps.
Schedule a demo to see how TLM manages your workflows, documents, and quality records!
2. ETQ Reliance
Image source: etq.com
ETQ Reliance is a cloud native system used in regulated industries. The platform includes more than 40 applications that support document control, training records, audits, CAPA, supplier activity, and risk programs.
It also supports groups that follow the FDA 21 Code of Federal Regulations (CFR) Part 11 rules for electronic records and signatures.
Teams often review ETQ Reliance when they want to cover a wide range of quality processes and offer configuration choices for different departments.
Key Features
- Audit planning, scheduling, and reporting
- Analytics dashboards that help teams analyze quality trends
- New product introduction tracking across the lifecycle
- Failure modes and effects analysis for risk review
- Supplier ratings and receiving records
- Non-conformance tracking and customer feedback
- Electronic signatures for regulated submissions
- Job safety analysis and incident reporting
- Enterprise risk surveys and compliance items
- Environmental management for permits and targets
Pros
ETQ Reliance gives teams a wide range of applications for quality and safety work, which helps larger groups keep these tasks in one place.
The codeless design environment makes it easier to adjust workflows when procedures change.
Teams also use the analytics dashboards to spot trends across modules. The mobile app helps field staff capture photos or notes during site activity.
Cons
Users said the interface looks dated in certain areas and takes extra clicks to reach settings. Integration with enterprise resource planning systems can require custom work, which adds time to setup.
Report building may be difficult for teams without database experience. The mobile view can feel limited for users who record frequent field notes.
Others mention that they want clearer API documentation and more filter controls for day-to-day use.
3. Qualio

Image source: qualio.com
Qualio is an electronic quality management solution used by life science companies that work in regulated environments.
It supports medical devices, pharmaceuticals, biotech, cannabis, and software as a medical device.
The platform manages documents, training, non-conformances, corrective and preventive action, audits, suppliers, and design work in one place.
It also supports regulatory compliance for FDA, ISO, and good practice (GxP) standards.
Organizations often look at Qualio when they want software options that organize quality records across multiple processes.
Key Features
- Document drafts, reviews, and controlled release
- Supplier records and classification
- Design control tracking across product stages
- Risk management aligned with ISO 14971 for medical devices
- Training assignments and completion records
- Change control with full traceability
- Audit planning and readiness tracking
- CAPA and nonconformance workflows
- Industry-specific functions for pharmaceuticals, devices, and biotech
Pros
Qualio offers a layout that users understand quickly and use with minimal guidance. It keeps quality activity connected, which helps teams maintain visibility across documents, training, and events.
Users can import several document formats during setup, which supports faster implementation for companies with existing records.
The platform also offers enough flexibility to adjust certain fields and forms to match internal procedures.
Cons
Users want quicker access to revision history without opening each file. Others note that manager-level visibility is limited unless they have administrator access.
Workflow automation is limited and affects certain document routes. Users who work with design controls and risk records also report inconsistent behavior when risk codes change.
This can affect ISO 13485 and ISO 14971 alignment.
4. MasterControl
Image source: mastercontrol.com
MasterControl is a QMS for life science organizations. It supports medical device, pharmaceutical, and biotech teams that manage documents, training, audits, quality events, and risk activity.
The platform also extends into manufacturing with electronic batch records, electronic device history records, work instructions, and logbooks.
MasterControl uses an AI management system that holds ISO 42001 certification. This confirms that its AI controls meet defined requirements for transparency and security.
Key Features
- Rules-based routing that automates steps based on set conditions
- Document control with versioning, eSignatures, and archiving
- Automatic document translation and AI-generated change summaries
- Training management with scheduling and competency tracking
- Exams that launch from document updates, CAPAs, and production records
- Risk management tools for identifying and analyzing hazards
- Audit planning and audit execution support
- Dashboards and reporting for trend review
- Validation tools that shorten setup time
- Support for electronic batch records and device history records
Pros
MasterControl Quality Excellence offers a wide set of functions for regulated work across quality and manufacturing.
Some users highlight its strong document control and detailed audit trails. They also mention that updates reflect customer feedback.
Cons
According to users, the interface looks dated and takes time for new staff to learn. Others say certain workflows require many steps.
Customization may require vendor involvement, which can slow setup.
Multiuser editing is limited, and some teams want more updates for the document and training modules. System performance may slow down during heavy use.
5. Arena

Image source: arenasolutions.com
Arena manages product information for electrical, mechanical, and software components in one record.
It connects quality activity to the product design, which helps teams track device master records (DMRs), design history files (DHFs), and CAPAs.
The platform supports new product development and new product introduction. It centralizes reviews and reduces confusion during release work.
Companies in regulated markets use Arena to maintain traceability and support compliance work tied to training, documents, and inspections.
Key Features
- Quality processes connected to engineering changes
- Closed-loop CAPA workflows
- Training management with tracked progress
- Issue and change management tools
- Supplier collaboration with controlled access
- Support for requirements and software validation tasks
- Cloud access that allows distributed teams to work from any location
Pros
Users often mention that Arena links product and quality information clearly. Several teams report better collaboration once the system is implemented.
Others appreciate the quick responses from the support team. Validation documentation also helps companies that work under strict regulatory expectations.
Cons
Many users feel the pricing is high for their business. Some find the navigation confusing since the menus have many options.
Others mention that the search function requires exact item numbers, which slows daily work. Several teams want more flexible edit options and a way to remove items to keep their workspace organized.
Import and multi-edit actions also feel slow compared to other QMS software options.
Why TLM Outperforms All Other Greenlight Guru Competitors

TLM solves the main issues companies report when they leave Greenlight Guru.
The dual application structure separates detailed quality work from routine tasks. Managers use the main application for workflow setup and record development.
Staff use the web app for reviews, approvals, and updates. This layout keeps responsibilities organized and helps teams maintain accuracy without slowing down daily work.
You won’t experience the same limitations seen in other platforms. TLM includes quality, production, maintenance, training, inventory, and risk modules in one environment.
Teams access connected data instead of switching between several systems. Deployment options and integration support help companies keep their existing structure without major adjustments.
The pricing model includes all modules along with validation materials and template procedures. Companies avoid extra charges and unpredictable upgrades since everything is included at the start.
Set up a demo and review your quality tasks inside TLM with a product expert. Activate the 30-day supported trial to test the platform with your own records!
FAQs About Greenlight Guru Competitors
What is Greenlight Guru used for?
Greenlight Guru is an electronic quality management system for medical device companies. It helps teams manage documents, risk files, design controls, audits, and training.
Organizations use it to support FDA and ISO requirements and keep records ready to submit during regulatory reviews.
What is the difference between Qualio and Greenlight Guru?
Qualio serves a wider set of life science organizations. It supports document control, supplier activity, training, and risk tasks in one environment.
Greenlight Guru focuses on device development and offers structured areas for design controls and quality events.
Buyers often compare them during early sales conversations to see which one helps them improve daily work without complex processes.
What is the best Greenlight Guru alternative?
TLM is often viewed as the best option for teams that want deeper customization and broader coverage across quality, production, and training. Some groups explore free alternatives during early research.
Most regulated companies eventually choose a system that supports audit readiness and handles quality tasks with consistent speed.






 Demos
Demos