Implementing a laboratory quality management system compliant with ISO 17025 has never been easier. Total Lean Management (TLM) QMS Software provides all the workflows needed to get your laboratory QMS implemented and audit ready smoothly and efficiently.
While ISO 17025 contains many of the same requirements of ISO 9001, a laboratory quality management system contains some unique requirements.

4 General Requirements
4.1 Impartiality
When your lab is being audited for its compliance to the requirement that it remains impartial, what are you going to tell the auditor that is convincing in terms of your responsibility to identify and manage risks associated with the requirements in this section? Total Lean Management (TLM) QMS Software makes this easy by linking individual employee records with the integrated Risk & Opportunity module.
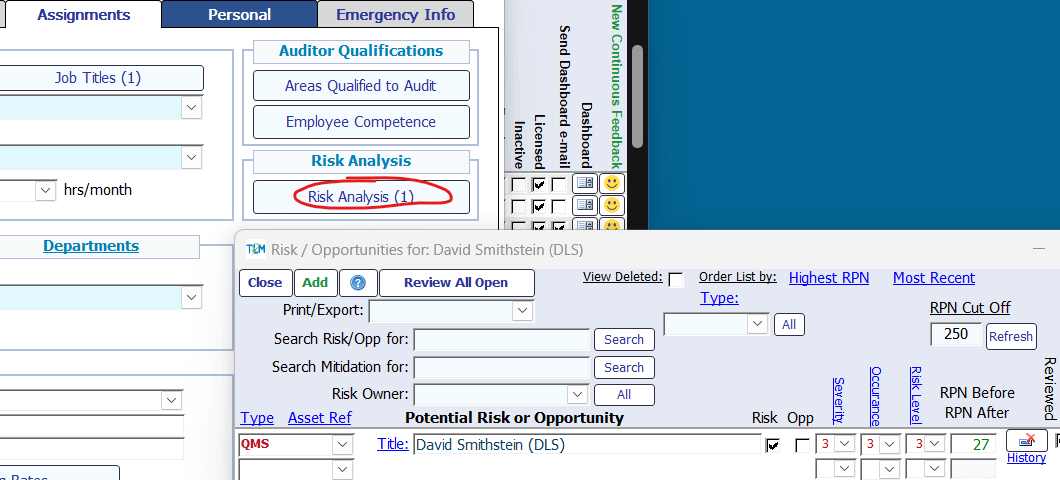

5 Structural Requirements
Conformance to the requirements in this section are supported by several modules, including Document Management, Deviations, Meetings & Reviews, and Employee Information.
In addition, the requirements in section 5.5 can be addressed with the Job Description documents that are linked to each employee, as well as establishing the relationship between employees and their immediate supervisors.
6 Resource Requirements
6.2 Personnel
Building your laboratory quality management system for ISO 17025 with TLM has many advantages built into the software from years of customer feedback identifying all the details that allow efficient conformance to this standard.
For example, the Training Module in TLM in combination with job description and competency features in the Employee Information module allows both a flexible and comprehensive approach to managing employee qualification, training, technical knowledge, skill and experience.
6.3 Facilities and Environmental Conditions
The equipment module includes environmental data in order to conform to requirements of laboratory conditions that are documented in the appropriate areas of TLM.
6.4 Equipment
The equipment module also includes events for maintenance as well as inspection activities.
Attachments can be made to any record in TLM in order to document external calibration records, for example.
7 Process Requirements
7.1 Review of requests, tenders and contracts
Total Lean Management (TLM) QMS Software also has a built in CRM Module with both self contained contract review capability, but can also be integrated with other online CRM systems, such as ZOHO One, so that the best approach to maintaining records of contract reviews and customer interactions is easy for everyone involved in the laboratory quality management system for ISO 17025.
7.2 Selection, verification and validation of methods
When internal procedures can reference externally published standards or methods, TLM has a variety of tools to help set up those links in order to make it convenient for employees who perform the work controlled by this documentation. This includes:
- Links can be set up to both external hyperlinks as well as other documents controlled by the laboratory quality management system that the web app displays in the browser, without having to download uncontrolled copies of the document.
- External standards and methods can be listed in the audit module, and then linked to individual procedures.
- If external documents are maintained and updated outside of the Laboratory QMS, TLM can use external links in the release of these standards. Periodic reviews can also be scheduled to maintain the accuracy of these reference links.
7.5 Technical Records
Maintaining adequate technical records for each laboratory activity can be challenging to keep organized. TLM allows multiple attachments to each quality record, that can either be embedded into the MySQL database, or linked to a network shared drive or online record.
This flexibility allows the laboratory to deploy the best tool for the job, as well as allowing TLM to maintain the overall digital landscape of the laboratory quality management system for ISO 17025.
7.8 Reporting of Results
Multiple reports are available for each module, both for individual records, such as equipment calibration, but as well as trending data for each module and live SPC data.
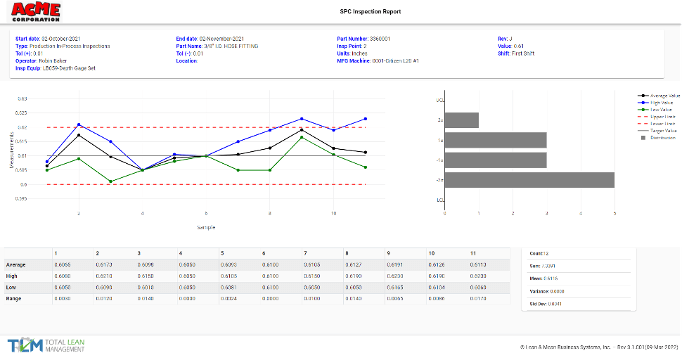
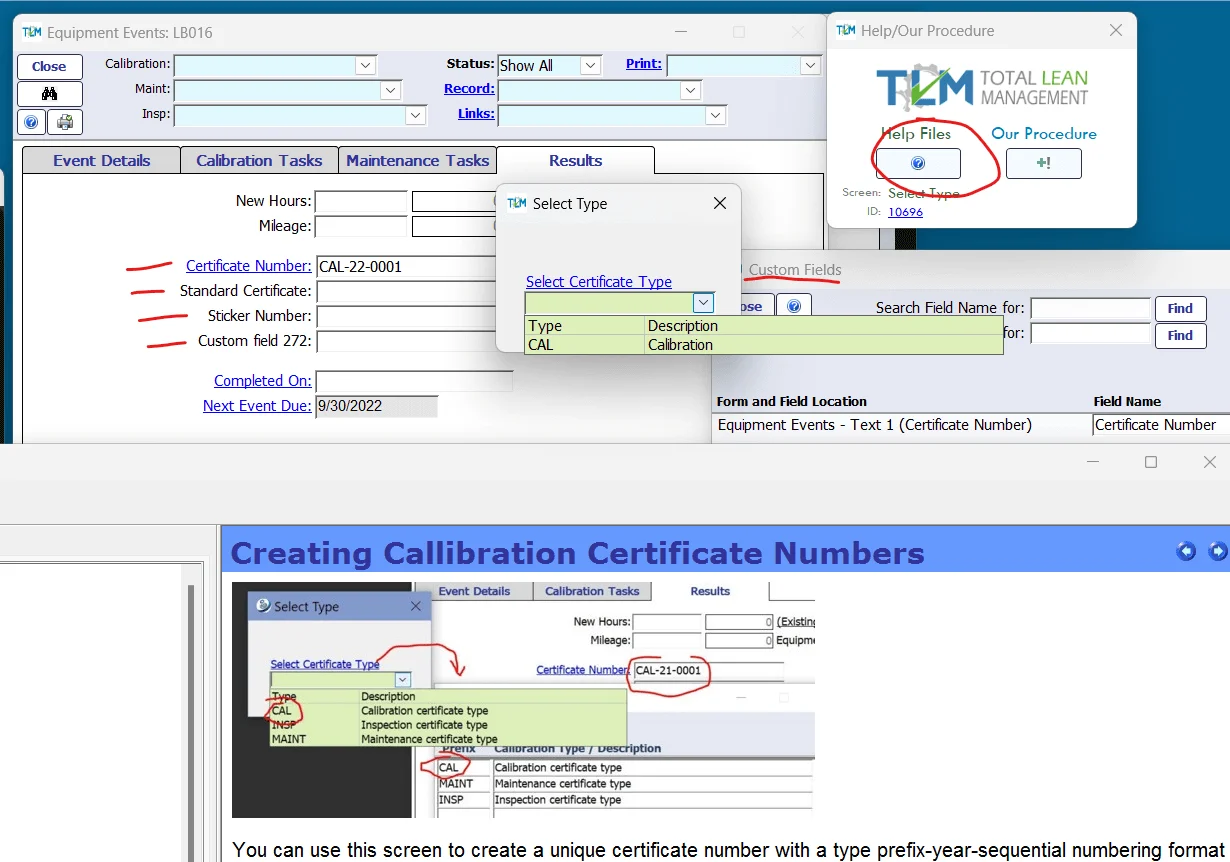
7.8.4 Specific requirements for calibration certificates
TLM supports the creation of unique certificate numbers, as well as a custom field system in order to label the types of certificates or stickers issued.
Integrated help files can display both standard help information, but the laboratory can also link their own internal procedures and work instructions to individual TLM screens to help guide users when using Total Lean Management (TLM) QMS Software for their laboratory quality management system for ISO 17025.
7.9 Complaints
The Customer Feedback module in TLM provides a mean to receive, evaluate, and make the needed decisions about customer feedback and complaints.
7.10 Nonconforming Work
The Rejected Materials module in TLM or the Deviations module can be implemented when any aspect of its laboratory activities or results of this work do not conform to its own procedures or the agreed requirements of the customer.
7.11 Control of Data and Information Management
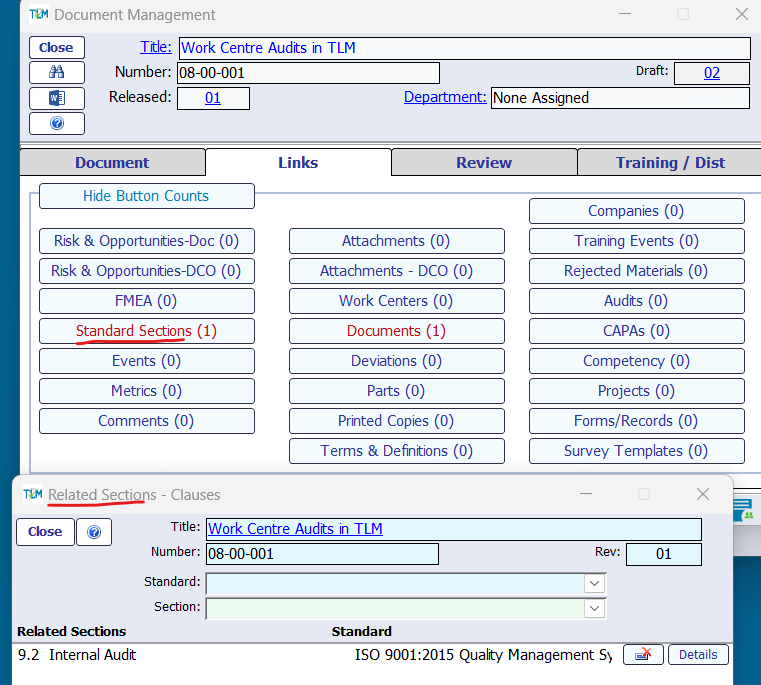
Total Lean Management (TLM) QMS Software accels at helping you set up a laboratory quality management system for ISO 17025 by interlinking to your controlled documents released in the Document Management Module.
As the cornerstone of any quality management system, TLM supports document links to over 24 different relationships to other TLM modules or record types.
8 Management System Requirements
8.1.2 Option A
Whether taking the approach of Option A or Option B, TLM has all the QMS workflows to provide flexible, risk appropriate management of these required activities:
- management system documentation (see 8.2);
- control of management system documents (see 8.3);
- control of records (see 8.4);
- actions to address risks and opportunities (see 8.5);
- improvement (see 8.6);
- corrective actions (see 8.7);
- internal audits (see 8.8);
- management reviews (see 8.9).
8.1.3 Option B
A laboratory that has established and maintains a management system, in accordance with the requirements of ISO 9001, and that is capable of supporting and demonstrating the consistent fulfillment of the requirements of Clauses 4 to 7, also fulfills at least the intent of the management system requirements specified in 8.2 to 8.9.





 Demos
Demos