What happens when regulators question your risk decisions? They expect records that show who evaluated the risk and what action followed.
Quality risk management software captures those decisions and applies them across operations. In regulated industries, the result influences audit outcomes, product approvals, and market access.
This article compares the leading quality risk management software options and shows which systems perform when risk reviews extend across the organization.
TL;DR
These are the five quality risk management software tools worth considering in 2026:
- TLM
- Scilife
- SimplerQMS
- AmpleLogic
- Qualityze
Why Quality Risk Management Software Matters More Than Ever
Quality risk management software matters now as regulatory requirements demand risk-based thinking across quality management processes.
Quality leaders and regulatory managers are expected to show how potential risks are identified, assessed, and documented as work happens.
As per a U.S. Food and Drug Administration report, the FDA is now aligning medical device and life sciences requirements with ISO 13485. That shift raises expectations for patient safety and product quality.
Risk management activities now reach into development processes, audits, and the supply chain.
Quality risk management software helps quality and operations leaders manage risk across the entire organization.
Risk assessment connects to quality processes, and records show how identified risks are addressed before audit findings or product issues appear.
Top 5 Tools for Quality Risk Management in 2026
Each quality risk management software platform handles risk differently. The comparison below shows how leading systems manage risk during audits, validation, supplier reviews, and change management.
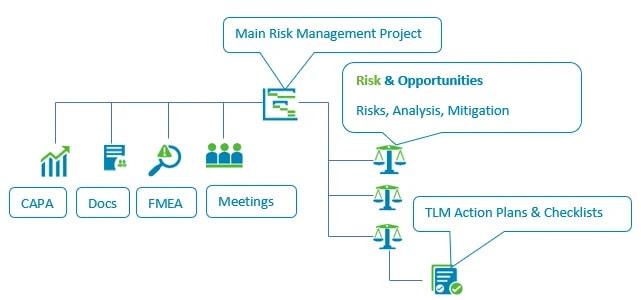
1. TLM

Total Lean Management (TLM) is a quality management system (QMS) that includes a Risk and Opportunities module for quality risk management.
It’s used in regulated industries such as manufacturing and medical devices, where documented risk decisions face regulatory review.
TLM connects risk management to audits, corrective and preventive action (CAPA), validation, suppliers, complaints, and change records.
Key Features
- Risk and Opportunities module used across the QMS
- Failure Mode and Effects Analysis with risk priority number (RPN) scoring
- Integrated FMEA and Control Plans to facilitate PPAP
- Two-factor or three-factor RPN calculations
- Mitigation scoring with approvals
- Risk management projects with linked records
- Coverage for ISO 14971, ISO 31000, ISO 9001, IATF 16949, and ISO 27001
Risk Assessment Within Quality Processes
TLM allows users to launch risk assessments from audits, CAPA, changes, suppliers, validation, and complaints.
Each record captures failure modes, effects analysis, and RPN values based on severity, likelihood, and detection scores.
Risk records connect to root cause analysis and corrective actions. This shows how mitigation reduces residual risk and addresses quality issues.
Project-Based Risk Management
TLM includes a Projects module used for managing organized risk management efforts. Projects link risk records, approvals, mitigation actions, and asset references under one plan.
The same module supports IT security and information risk under ISO 27001, including asset tracking and approval workflows.
Dashboards, Tracking, and Daily Use
Risk records and mitigation tasks appear on user dashboards. Summary emails highlight priorities without manual follow-up.
Custom RPN color codes and mitigation cost-tracking help identify risk trends across the organization.
Multi-location options allow record separation where required. Data from external systems can flow into TLM through the Connection Manager when quality records depend on other databases.
Configuration and Validation Use
TLM allows configuration through system settings, custom fields, and custom forms with email notifications and approvals. Field names and module labels can match internal terminology to support training.
Medical device companies use TLM validation documentation templates to support ISO 13485 certification.
TLM also offers AI support for queries against released documents and for compliance reviews of individual documents.
Schedule a demo to see how TLM handles quality risk management across your organization!
2. Scilife
Image source: scilife.io
Scilife is used by medical device teams that want to manage quality risk assessment within a single system and keep records ready for review.
The Risk Assessment module allows users to create risks, complete risk analysis, and move records through review and approval stages.
Risk records connect with deviations, nonconformities, CAPAs, and audits, which keep risk management connected to active quality processes.
Scilife also supports recurring risk reviews, with scheduled assessments and version history that help teams revisit operational risks across the product lifecycle.
Key Features
- Risk Assessment module within the QMS
- Manual or automated risk scoring
- Predefined questions for consistent evaluations
- Risk links to deviations, nonconformities, CAPAs, and audits
- Scheduled recurring risk reviews
- Version history for risk records
- Centralized risk records across quality modules
Pros
Scilife is easy for users to adopt. Risk identification and quality risk assessment stay connected to other quality records, which helps teams mitigate risks during audits and reviews.
Cons
Users report concerns with system validation and data integrity. Support responses may close issues before full resolution, which can be a concern for organizations with strict regulatory expectations.
3. SimplerQMS
Image source: simplerqms.com
SimplerQMS is an eQMS for life sciences that manages risk through controlled documentation. Users manage risk assessments, risk analysis, and risk management plans inside the document control system.
Risk documents link to standard operating procedures (SOPs), training records, CAPAs, nonconformances, deviations, suppliers, and audits. This keeps risk documentation connected to quality records across business processes and product lines.
Electronic workflows handle review, approval, and scheduled reassessment of risk documents. Traceability matrices link risk mitigation strategies to related quality records.
This helps demonstrate risk-based decisions across the product lifecycle and meet industry standards.
Key Features
- Document linking across CAPA, audits, training, suppliers, and products
- Electronic workflows for review and approval
- Traceability matrix templates with version tracking
- Scheduled risk review actions with notifications
- Electronic signatures compliant with FDA 21 CFR Part 11 and EU Annex 11
- Coverage for ISO 13485, ISO 14971, ISO 9001, GMP, ICH Q9, EU MDR, and IVDR
Pros
SimplerQMS is used by organizations that depend on strict documentation and traceability. GAMP 5 validation with ongoing revalidation supports regulatory expectations in life sciences.
Risk records are also easy to retrieve during ISO audits.
Cons
Risk management remains document-focused. Teams with highly configurable or niche workflows may encounter limits during setup. Users report usability issues when managing complex product or supplier relationships.
4. AmpleLogic
Image source: amplelogic.com
AmpleLogic provides a quality risk management solution for regulated industries to proactively identify risk.
The software supports risk identification and assessment so organizations can ensure compliance while addressing quality issues earlier in their processes.
The system allows teams to identify potential risks across processes, products, and systems, then evaluate those risks based on quality and compliance exposure.
Root cause analysis helps trace quality issues back to underlying factors, while built-in documentation and reporting support regulatory review.
Key Features
- Risk identification throughout processes, products, and systems
- Risk assessment to evaluate quality and compliance exposure
- Risk mitigation strategies and controls for proactive risk management
- Documentation and reporting for regulatory review
- Continuous monitoring of risk indicators and key metrics
- Cross-department collaboration on risk management activities
Pros
AmpleLogic covers the full risk management lifecycle in one system. Users report that technical support is responsive during implementation.
Cons
Users mentioned that the interface looks dated and requires extra clicks to complete tasks.
New users may need more time to find features, as the system offers limited in-app guidance and shortcuts during the early stages.
5. Qualityze
Image source: qualityze.com
Qualityze offers an integrated risk management system that helps organizations document risk assessment and mitigation work.
It focuses on risk identification, risk profiling, and centralized records so teams can understand their risk landscape and apply consistent risk management strategies.
Qualityze uses a central database to map risks and capture details such as risk type, source, and potential impact.
Teams set evaluation criteria like severity and likelihood, then use a custom risk matrix to prioritize risks. Risk thresholds flag items that exceed acceptable levels, which speeds risk reduction planning.
Qualityze tracks mitigation through risk response plans. Users can route actions through workflows, trigger CAPA, adjust processes, or add preventive plans.
Key Features
- Centralized risk mapping with risk profiles
- Risk evaluation criteria for severity and likelihood
- Custom risk matrix and risk thresholds
- Risk response plans, CAPA, and preventive actions
- Investigation tools for root cause analysis and recurrence tracking
- Centralized records, dashboards, reporting, and risk trends
- Integration with document management, CAPA, and change management
Pros
Qualityze integrates risk management with core quality processes. Some users report fast response times from the implementation team and helpful support with custom configurations.
Cons
Qualityze typically starts with a sales demo rather than a self-serve trial.
Users also mention that trend graphics for repeated inconsistencies aren’t generated automatically, so they rely on other tools for quarterly trending.
Test Quality Risk Management Workflows With TLM
Many quality risk management tools record risk decisions but do not carry them to different business units. Risk information remains limited to specific areas, creating uneven coverage across industries and leaving gaps during audits or investigations.
TLM operates as an integrated solution that keeps risk management consistent. Risk decisions remain connected wherever quality work occurs, which helps improve product quality as operations expand.
TLM supports enterprise-wide use without forcing every team into the same habits. Risk management adapts as requirements change, without requiring a reset.
FAQs About Quality Risk Management Software
What is the quality risk management tool?
A quality risk management tool is software used to identify, assess, and document risks that affect product quality and compliance. It records risk decisions, mitigation actions, and approvals in one system.
What is the best risk management software?
There is only one type of quality management system, yours. Therefore, the best QMS software is the one that can best adapt to how your QMS should work at your company.
Many organizations consider TLM when they need more control over approvals, records, and audits, as TLM is probably the best at adapting to customer needs using system settings, custom fields, the new custom form builder.
It also offers free and sponsored update projects that add specific capability to either the main app, web app, or both TLM applications as part of our Rapid Update support capability.
For regulated industries, TLM is often selected for its integrated quality risk management capabilities.
What KPIs are used for risk management?
Risk management KPIs track how well risks are identified, reduced, and reviewed. Common measures include open risks, residual risk after mitigation, and how long it takes to close risk actions.
Organizations also monitor recurring risks, overdue actions, completion of scheduled risk reviews, and overall risk trends to confirm that controls remain effective.





 Demos
Demos